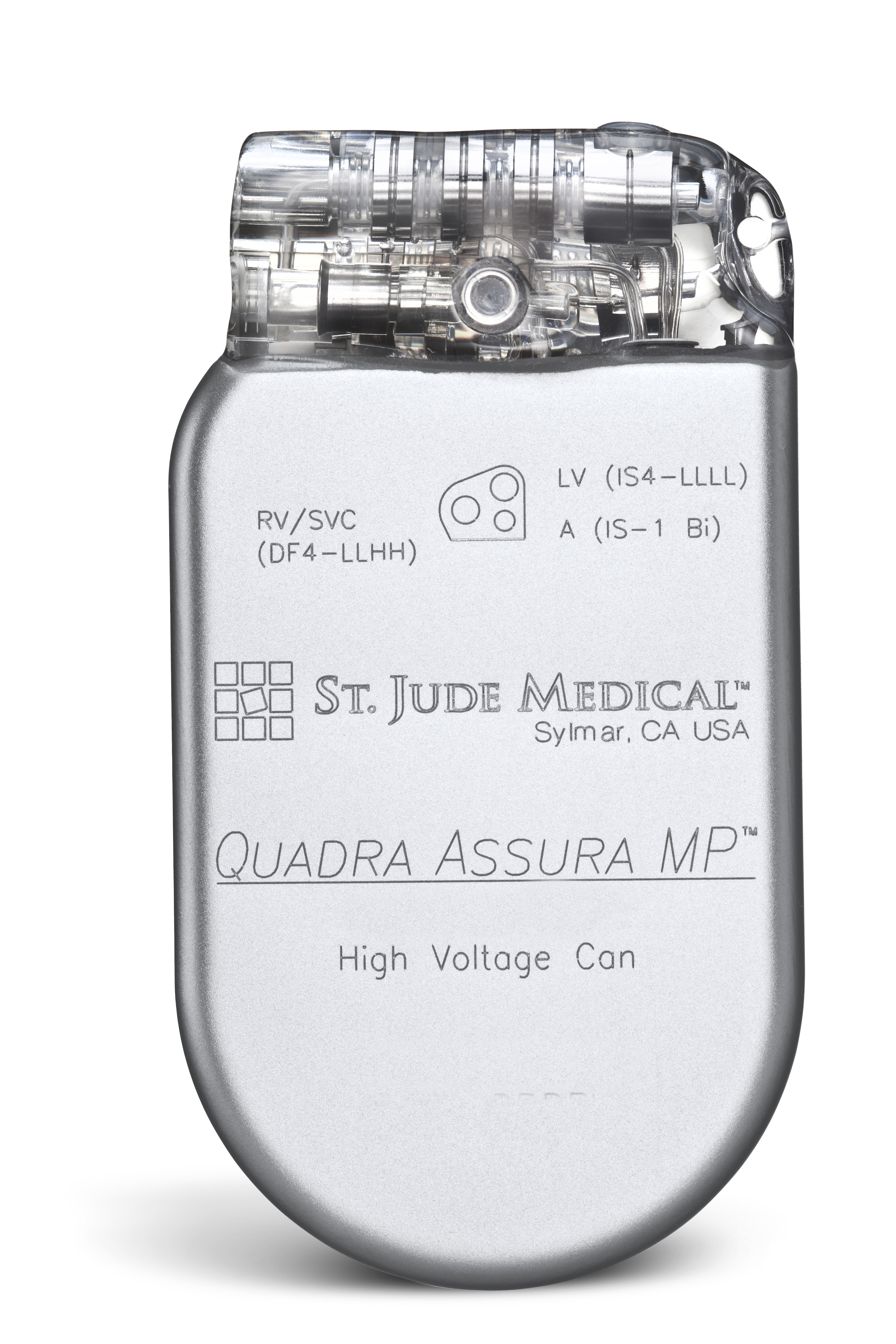
St Jude Quadra Assura Mp
Device Problem Over-Sensing 1438 Patient Problem No Known Impact Or Consequence To Patient 2692 Event Date 01272015. In addition to the approval of SyncAV CRT software the company has also received CE Mark approval for the Quadra Assura MP.

Defibrillator Buy Defibrillator United States From St Jude Medical Find Here More Details About The Seller And Other Related Products Id 3308716
Jude Medical MR Conditional ICD system is conditionally safe for.

St jude quadra assura mp. Back to Search Results. The medical device QUADRA ASSURA CD3367-40QC is realized by ST. Quadra Assura MP CD3371-40 CRT-D with RF telemetry DF-1IS-1 IS4-LLLL 40 J Untested Quadra Assura MP CD3371-40C CRT-D with RF telemetry Parylene coating DF-1IS.
Testing has demonstrated that the St. Meets industry-standard MRI testing requirements. In October 2016 St.
JUDE MEDICAL INC CRMD QUADRA ASSURA MP CRT-D IMPLANTABLE CARDIOVERTER DEFIBRILLATOR. Jude Medical has received CE mark clearance for its quadripolar device the Quadra Assura MP cardiac resynchronisation therapy defibrillator CRT-D. In Commercial Distribution Catalog Number.
Manufacturer Parent Company 2017 Abbott Laboratories. CD3265-40 CD3265-40Q CD3365-40C CD3365-40Q. Jude Medical EEUU antes del 23 de mayo de 2015 debido al posible agotamiento prematuro y repentino de la batería en un plazo que puede oscilar entre.
QUADRA ASSURA MP Version or Model. Jude Fortify Fortify Assura Quadra Assura Quadra Assura MP Unify Unify Assura Unify Quadra ICDs manufactured before May 23 2015. QUADRA ASSURA MP CD3371-40QC.
Like the previously released Quadra Assura the MP model takes advantage. Manufacturer of the medical device. The Quadra Assura MP CRT-D which is based on the companys Quadripolar Pacing System features MultiPoint Pacing MPP technology that allows physicians to pace the multiple locations on the left side of the heart.
Capable of full body 15T MRI imaging scans. CD3369-40Q Commercial Distribution Status. St Jude Medical Inc 15900 Valley View Ct Sylmar CA 91342-3577.
The Quadra Assura MP CRT-D and Quartet quadripolar LV pacing lead feature four pacing electrodes and 10 pacing vectors providing more options and greater control to minimize implant complications such as diaphragmatic. QUADRA ASSURA MP CRT-D ADVANCED FEATURES AND BENEFITS Now MRI Ready. Quadra Assura MP CRT-D.
Quadra Assura Model Numbers. QUADRA ASSURA MP CD3371-40QC. Jude Medical ICDs and CRT-Ds are intended to provide ventricular antitachycardia pacing and ventricular defibrillation for automated treatment of life -threatening ventricular arrhythmias.
Back to search results. Results of St Jude Medicals MultiPoint Pacing investigational device exemption clinical study have been presented during a late-breaking clinical trial session at the Heart Rhythm Societys HRS 37th annual scientific sessions. NYSESTJ a global medical device company today announced CE Mark approval of its next-generation quadripolar device the Quadra Assura.
CRT-D with RF telemetry Parylene coating DF4-LLHHIS4-LLLLIS-1. St Jude Medical Inc 15900 Valley View Ct Sylmar CA 91342-3577 Manufacturer Parent Company 2017. Quadra Assura MP CD3271-40Q CRT-D s RF telemetriou DF4-LLHH IS4-LLLL IS-1 40 J Tieto zariadenia možno programovať pomocou systému starostlivosti o pacienta Merlin so softvérom model 3330 verzia 142 alebo novšia.
The Quadra Assura MP CRT-D is MR conditional when used with specific leads and lead lengths and allows full-body 15T MRI scans that meet certain scan conditions. Quadra Assura MP Model Numbers. Jude medical inccrm-sylmar quadra assura mp icd implantable cardioverter defibrillator.
This device has been tested for safe performance of an MRI scan using a 15 T Tesla field-strength MRI scanner. Jude Medical MR Conditional Systems. St Jude Medical Quadra Assura MP.
Event Type Malfunction Event. JUDE MEDICAL symbol deviatich štvorcov a MORE CONTROL. The Quadra Assura MP CRT-D brings MultiPoint pacing an exclusive St.
Quadra Assura MP CRT-D is an MRI Ready device when used in combination with MR Conditional leads. Manufacturer of the medical device. Jude Medical won the European CE Mark for its Quadra Assura MP cardiac resynchronization therapy defibrillator CRT-D.
La AEMPS emite recomendaciones para el seguimiento de los pacientes implantados con los Desfibriladores Fortify Fortify Assura Quadra Assura Quadra Assura Mp Unify Unify Assura y Unify Quadra fabricados por St. CD3269-40 CD3269-40Q CD3369-40C CD3369-40Q Unify and Unify Assura cardiac resynchronization therapy defibrillators. Jude Medical technology to cardiac resynchronization therapy defibrillators CRT-Ds.
The medical device QUADRA ASSURA MP CD3371-40QC is realized by ST. 15T MR Conditional Systems DeviceLead Combinations for QuadraAssura and Quadra Assura MP CRT-Ds Device model Lead model lengths RF Power Scan regions Quadra Assura CD3367-40Q CD3367-40QC Quadra Assura MP CD3371-40Q. Quadra Assura MP CD3369-40QC.
The Quadra Assura MP is one of the CRT-Ds affected by the recall. Jude Medical recalled a subset of implantable cardioverter defibrillators ICDs and cardiac resynchronisation therapy defibrillators CRT-Ds due to reports of rapid battery failureTo address the issue the company has released a battery performance alert tool designed to warn patients and. OVERVIEW OF QUADRA ASSURA MP TM CRT-D.
Fortify ST VR Fortify Assura STVR CD1241-40 40Q CD1263-4040Q CD1363-40C 40Q Ellipse VR CD1411-36C CD1411-36Q CARDIOVERTER-DEFIBRILLATOR OTHER THAN SINGLE OR DUAL CHAMBER IMPLANTABLE C1882 Unify Assura CRT CD3357-40C CD3357-40Q Quadra Assura CRT CD3365-40C CD3365-40Q Quadra Assura MP CRT-D CD3369-40C CD3369-40Q.

Help Manual St Jude Medical Instructions For Use Website Manualzz
St Jude Gets Ce Mark For Ellipse Assura High Voltage Cardiac Devices Medgadget

New St Jude Medical Cd3359 40c Quadra Assura Mp Cardiac Resynchronization Device Tiered Therapy Cardioverter Defbrillator Disposables General For Sale Dotmed Listing 3593629

Samenvatting St Jude Medical Breidt Portfolio Hartfalen Therapieen Uit Met Syncav Crt Technology Extra Behandeloptie Voor Niet Responsieve Patienten Business Wire

St Jude Medical Touts 1st U S Commercial Implantations For Quadra Assura With Multipoint Pacing Massdevice
Samenvatting St Jude Medical Breidt Portfolio Hartfalen Therapieen Uit Met Syncav Crt Technology Extra Behandeloptie Voor Niet Responsieve Patienten Business Wire
St Jude Medical Touts 1st U S Commercial Implantations For Quadra Assura With Multipoint Pacing Massdevice

Battery Performance Alert Update On Class I Recall Of St Jude Medical S Icds And Crt Ds
Cardioversion Defibrillation Page 2 The World Of Implantable Devices

Abbott Announces Mr Conditional Labeling For Quadra Assura Crt D And Fortify Assura Icd Daic

Fda Bad Batteries On St Jude Defibrillators Run Out In 24 Hrs Daily Hornet Breaking News That Stings
St Jude Medical Launches New Study Of Next Gen Multi Point Cardiac Pacing Massdevice

Medical Equipments Pacing Lead Tendril Sts Wholesale Distributor From Pune

St Jude Recalls Defibrillators For Battery Hazard Daily Hornet Breaking News That Stings

Fda Approval Of Devices Today S Medical Developments

Posting Komentar untuk "St Jude Quadra Assura Mp"