Good Manufacturing Practices And Inspection Quality Assurance Of Pharmaceuticals
These Good Manufacturing Practices GMP for Active Pharmaceutical Ingredients API guidelines GUI-0104 are designed to facilitate compliance by the regulated industry and to enhance consistency in the application of the regulatory requirements. Quality assurance of pharmaceutical products is a continuing concern of WHO.
Read Good Manufacturing Practices Gmp Modules For Pharmaceutical Products Online By Chandrasekhar Panda Books
Good Manufacturing Practices and Inspection Volume 2 2007 which you can access in its entirety on that link if youre really serious about this kind of thing.

Good manufacturing practices and inspection quality assurance of pharmaceuticals. EMAs GMPGDP Inspectors Working Group is discussing actions required after an inspection concludes that a manufacturing site does not comply with GMP specifically where this can lead to a shortage of critical medicines. These guidelines provide minimum requirements that a manufacturer must meet to assure that their. 2 including updates.
Main principles1 Introduction 79 General considerations 80 Glossary 81 Quality management in the medicines industry. Good quality assurance managers are integral to the smooth operation of a quality assurance department. These managers use the principles ISO 9001 to ensure that quality documents that conform to the regulations are developed and maintained personnel are qualified and appropriately trained and all activities are conducted according to the appropriate corporate and regulatory standards.
QA is charged with preventing defects and errors from occurring by overseeing all. Good manufacturing practices GMP are the practices required in order to conform to the guidelines recommended by agencies that control the authorization and licensing of the manufacture and sale of food and beverages cosmetics pharmaceutical products dietary supplements and medical devices. We cannot guarantee that.
Vikash Kumar Chaudhari1 Vijay Yadav2 Praveen Kumar Verma1 Amit Kumar Singh2 1Department of Pharmaceutical Chemistry 2Department of Pharmacognosy Kunwar Haribansh Singh College of Pharmacy Jaunpur UP. Good manufacturing practices GMP are the practices required in order to conform to the guidelines recommended by agencies that control the authorization and licensing of the manufacture and sale of food and beverages cosmetics pharmaceutical products dietary supplements and medical devices. It aims to mitigate the risks that are inherent in the production process.
The following points are from a World Health Organization book titled Quality Assurance of Pharmaceuticals. Quality assurance of pharmaceuticals. To respond to the global need for adequate quality assurance of pharmaceuticals WHOs Expert Committee on Specifications.
2 Good manufacturing practices and inspection 2nd ed. Contract production and analysis 51 General 51 The contract giver 51 The contract accepter 52 The contract 52 8. A Compendium of Guidelines and Related Materials.
World Health Organization Quality assurance of pharmaceuticals. Quality assurance of pharmaceuticals 2019 WHO guidelines good practices related regulatory guidance and GXP training materials Quality assurance of pharmaceutical products is. Philosophy and essential elements 85 1.
The quality of pharmaceuticals is a continuing concern of the World Health Organization WHO. Qualification and validation 48 5. Quality assurance 45 2.
Current Good Manufacturing Practices CGMPs help to establish the foundation for quality pharmaceuticals through regulatory standards. Good manufacturing practices and inspection World Health Organization Geneva 2004 QAPPR 3 112103 0514 PM. Self-inspection and quality audits 53.
Good Manufacturing Practice for Products As a part of quality assurance good manufacturing practice is concerned with production and quality control. It should be noted that these guidelines do not cover safety aspects for the personnel engaged in the fabrication packaginglabelling and testing. Revision of template for serious GMP non-compliance.
There are three keys to pharmaceutical product quality control. Sanitation and hygiene 48 4. Its a fact.
A compendium of guidelines and related materials. The setting of global standards is requested in Article 2 of the WHO Constitution which cites as one of the Organizations functions that it should develop establish and promote international standards with respect to food biological pharmaceutical and similar products. Quality assurance of pharmaceuticals good manufacturing practices and inspection Download Quality Assurance Of Pharmaceuticals Good Manufacturing Practices And Inspection ebooks in PDF epub tuebl textbook from SkinvadersCom.
This report describes the roles of Good Manufacturing Practices GMP in pharmaceutical product quality control. CGMPs regulations enforced by FDA provide. Pharmaceutical quality system 85.
A compendium of guidelines and related materials. Read online Quality Assurance Of Pharmaceuticals Good Manufacturing Practices And Inspection books on any device easily. They are specifications thorough product characterization during development and adherence to GMP as the ICH Q6A guideline on specifications provides the most important principles in its background section.
WHO good manufacturing practices for pharmaceutical products. Good manufacturing practices for pharmaceutical products GMP 47 3. PDF On Jan 14 2019 Ali Alsamydai and others published Good Manufacturing Practices for Pharmaceuticals Find read and cite all the research you need on ResearchGate.
Its basic requirements according to WHOs Good Manufacturing Practices for Pharmaceuticals state the following. Good manufacturing practices guidelines gmp guidelines are integral part of any quality systems therefore failure of the quality assurance system or quality system is critical and damaging to the basis of the quality assurance in turn quality of drugs and medical devices manufactured and hence would not be affordable and acceptable by either manufacturer or regulatory agencies. Good-manufacturing-practice inspection report - Community format.
These guidelines provide minimum requirements that a manufacturer must meet to assure that their. 2 Good manufacturing practices and inspection 2018-02-23 Quality Systems Book or Program 1376 views Editor. A compendium of guidelines and related materials.
Pharmaceutical manufacturers must integrate the key functions of quality control which checks for defects after they have occurred and regulations or GMP under the banner of quality assurance. Product recalls 50 7. Despite efforts made around the world to ensure a supply of quality and effective medicines substandard spurious and counterfeit products still compromise health care delivery in many countries.
DOWNLOAD AS PDF ABOUT AUTHORS. RUNNING HEAD iv G WHO Library Cataloguing-in-Publication Data Quality assurance of pharmaceuticals.

Medicines Quality Assurance Who Normative Functions In The

The Role Of Quality Assurance In The Pharmaceutical Industry Youtube

Quality Assurance Of Pharmaceuticals A Compendium Of Guidelines And Related Materials Vol 2 Good Manufacturing Practices And Inspection

Difference Between Cgmp And Gmp Pharmaceutical Guidelines

Amazon Com Good Manufacturing Practices For Pharmaceuticals Seventh Edition Drugs And The Pharmaceutical Sciences Ebook Bunn Graham P Kindle Store

Ppt Good Manufacturing Practices Powerpoint Presentation Free Download Id 368931

Default Set Quality Assurance Of Pharmaceuticals Op A Compendium Of Guidelines And Related Materials 9789241547086 Medicine Health Science Books Amazon Com
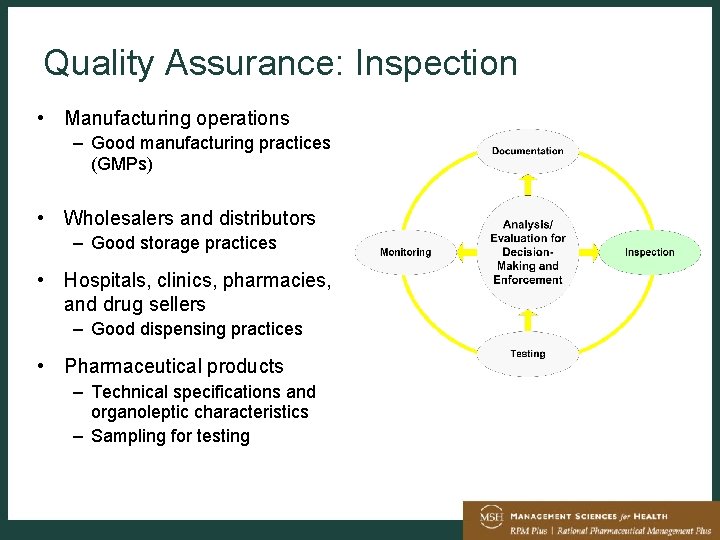
Drug Manufacture Quality Assurance And Regulation Tom Layloff

Quality Assurance Fundamentals In Good Manufacturing Practices Gmpsop

A Who Guide To Good Manufacturing Practice World Health

Good Manufacturing Practices For Pharmaceuticals A Plan For Total Quality Control From Manufacturer To Consumer M Dekker 2000 Webofpharma Com Pharmaceuticals Industry Web Of Pharma

Buy Good Manufacturing Practices And Inspection V 2 Quality Assurance Of Pharmaceuticals A Compendium Of Guidelines And Related Materials Book Online At Low Prices In India Good Manufacturing Practices And Inspection

Ppt Good Manufacturing Practices Purpose And Principles Of Gmp Powerpoint Presentation Id 287869

Quality Assurance Of Pharmaceuticals A Compendium Of Guidelines And Related Materials Vol 2 Good Manufacturing Practices And Inspection
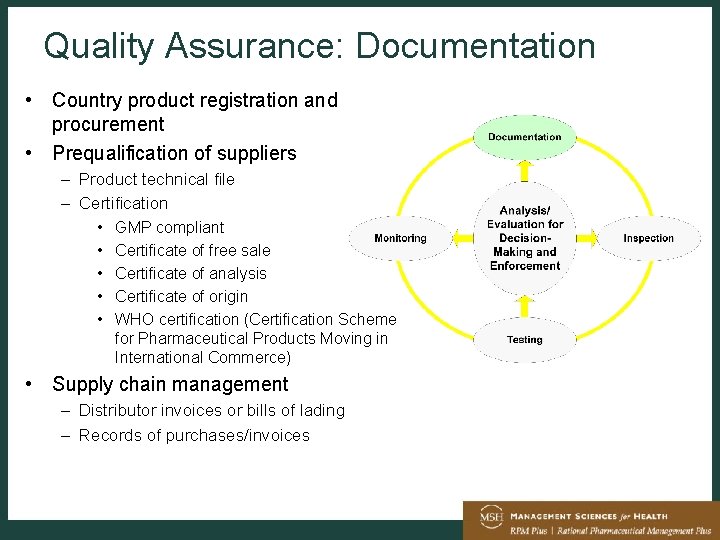
Drug Manufacture Quality Assurance And Regulation Tom Layloff

Gmp Guidelines Inspection Checklist For Cosmetics Good Manufacturing Practice Inspection Checklist Checklist
Quality Assurance Of Pharmaceuticals A Compendium Of Guidelines And Related Materials Vol 2 Including Updates Good Manufacturing Practices And Inspection

Assessment Of The Good Manufacturing Practices Inspection Program Of The Bangladesh Directorate General Of Drug Administration Siaps Program

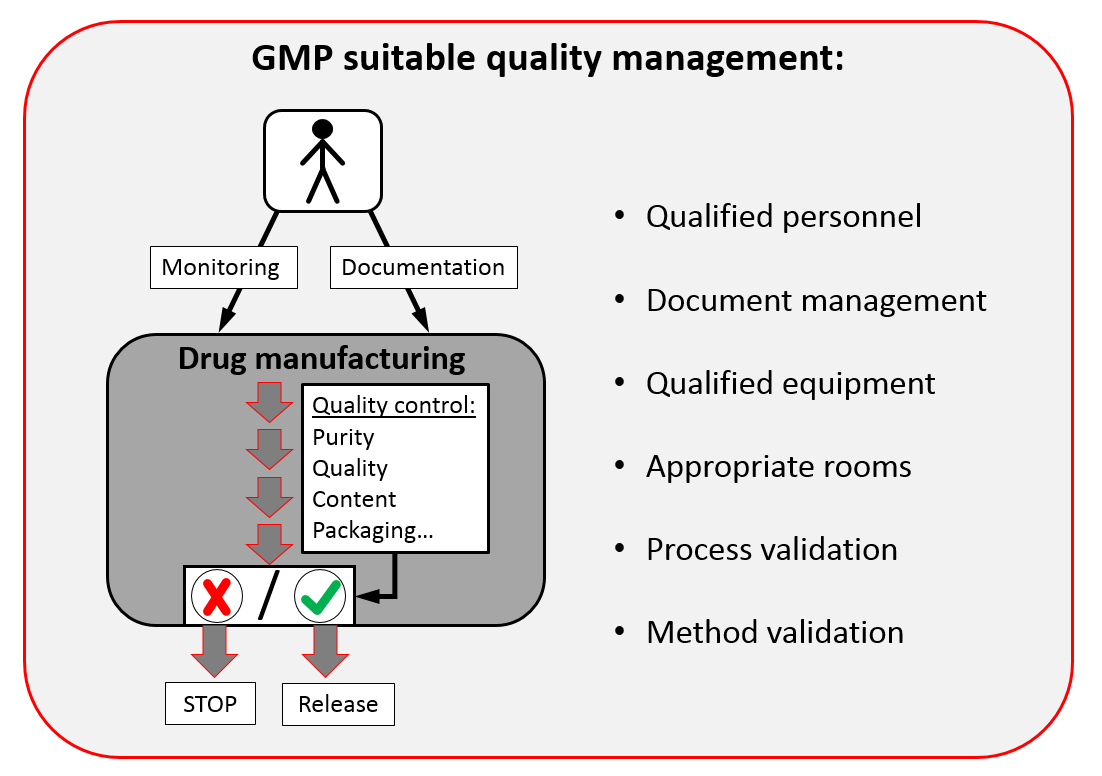
Posting Komentar untuk "Good Manufacturing Practices And Inspection Quality Assurance Of Pharmaceuticals"