Fda Computer System Assurance
The FDA draft guidance on Computer Software Assurance is a paradigm shift from document focused computer system validation to critical thinking assurance practices. How to accelerate adoption of FDAs Computer Software Assurance.
Fda Computer System Software Validation What You Ve Known For 20 Years Is Changing
FDA COMPUTER SYSTEM SOFTWARE VALIDATION WHAT YOUVE KNOWN.
Fda computer system assurance. GAMP 5 FDA CSA and the Future of Computer Systems Validation Webinar Extended Learning Learning Level. FDAs Transition From Computer System Validation To Computer Software Assurance. In addition computer systems used to create modify and maintain electronic records and to manage electronic signatures are also subject to the validation requirements.
Matlis President and Ellyn McMullin Research Associate. Computer Software Assurance CSA is a Case for Quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization and innovation. The FDA regulation 21 CFR Part 11 in 1997 and the related guidance of 2003 paved the road to implementation of computer system validation CSV by life sciences companies.
In 2019 FDA will be releasing a new draft guidance Computer Software Assurance for Manufacturing Operations and Quality System Software that embraces modern thinking on the topic. The FDAs general view of automation is basically a green light for companies. See 21 CFR 11.
The past of Computer Systems Validation has served us well and provided us with the foundation of where we are today. In fact FDA has taken the lead with a proposed guidance on Computer Software Assurance and is asking firms to rethink their approach. This new draft guidance will replace the term Computer System Validation with a new term Computer Software Assurance in an effort to have companies think critically about how software quality assurance is achieved using risk-based methodologies to justify the amount and types of testing required as well as leveraging testing completed during the SDLC process.
Computer Software Assurance for Production and Quality System Software Fostering Medical Device Improvement. But at some point the past can become a burden. In September of this year the FDA plans to release guidance on the transition from the traditional concepts of Computer System Validation CSV to Computer System Assurance CSA for Manufacturing Operations and Quality System SoftwareThe new guidance will provide clarity on the procedure used to determine what is high-risk and therefore what should be tested more stringently.
Establishment of documentary evidence that provide a high degree of assurance that a planned process will be uniformly in accordance with the expected specified results. About the Author Darren Geaney is a Process Engineer with over 20 years experience in Quality Assurance specializing in Computer System Validation. As pharmaceutical companies perfected their business processes and became more efficient in.
The FDA recognizes the value of using advanced technologies to enable the industry to make medicines of more reliable quality. This approach encourages critical thinking based on product and process knowledge and quality risk management over prescriptive documentation-driven approaches. The US FDA Center for Devices and Radiological Health CDRH Case for Quality program promotes a risk-based product qualityfocused and patient-centric approach to computerized systems.
FDA supports and encourages the use of automation systems and digital technologies throughout the product lifecycle to improve patient outcomes and reduce the risk to patients. I am looking forward to this guidance because in my experiences Ive made and observed decisions regarding automation and technology that were often hampered by fear and pain of validation. Computer Software Assurance CSA Computer system validation CSV is a much more familiar term than CSA but CSA is the wave of the future in validating your software.
Regulatory guidance on Computer System Validation CSV has remained mostly unchanged for the past two decades. The guidance was initially expected. Computer system validation has been around for a while certainly since the introduction of the FDA regulation 21 CFR part 11 in 1997.
Auditors such as the FDA require evidence and records therefore the CSV methodology inspires a compliance-mindset rather than an. This blog is the first of a three-part series focused on FDAs Computer Software Assurance CSA. For example take a look an excerpt below from the FDAs Principles of Software Validation Section 47.
NOM FDA WHO. FDA supports and encourages the use of automation systems and digital technologies throughout the product lifecycle to improve patient outcomes and. Set for release in the fiscal year 2020 the FDA has drafted up a guidance to Computer Software Assurance for Manufacturing Operations and Quality System Software to address the pain points in the following ways.
Computer System Assurance. The agency encourages the use of automation. The Guidance is on FDAs list for release in September 2020 and applies to non-product quality system software solutions.
This is exactly the point in time where we have arrived. The focus of current CSV processes is producing accurate and approved documentation to present information to auditors. Food and Drug Administration FDA is expected to release the Computer Software Assurance for Manufacturing and Quality System Software guidance in 2021.
The FDA outlined a. As always this framework is acceptable today under current guidelines and the FDA is encouraging the industry to adopt it even prior to release. Extended Learning Webinars take a 90-minute deeper dive into industry-critical topics you want and need to learn more about.
The FDA is expected to release its new guidance around CSA Computer Software Assurance for Manufacturing Operations and Quality Systems Software before the end of 2020. This Computer Systems Validation Guide is based on the following approaches. Food Drug Administration FDA on how to ensure your systems are operating as required.
Matlis President and Ellyn McMullin Research Associate. Hello Computer System Assurance CSA. If youve ever managed a computer software validation project youre undoubtedly familiar with the notably ambiguous guidance from the US.
FDA Activities and Engagement with the Voluntary Improvement Program. Technology on the other hand has changed significantly with cloud first architectures and broad adoption of 21 CFR Part 11 compliant solutionsThe FDA is bridging this gap between regulation and technology through its upcoming guidance Computer Software Assurance for. Hello Computer System Assurance CSA.
With FDAs heightened focus on Software Assurance and Data Integrity Computer Systems Validation has taken on a new movement initiated by regulatory authorities.

Computer Software Assurance Csa The New Csv

Computer Software Assurance Risk Assessment Takes Center Stage

Computer Software Assurance Csa The Fda S New Approach To Csv Kalleid
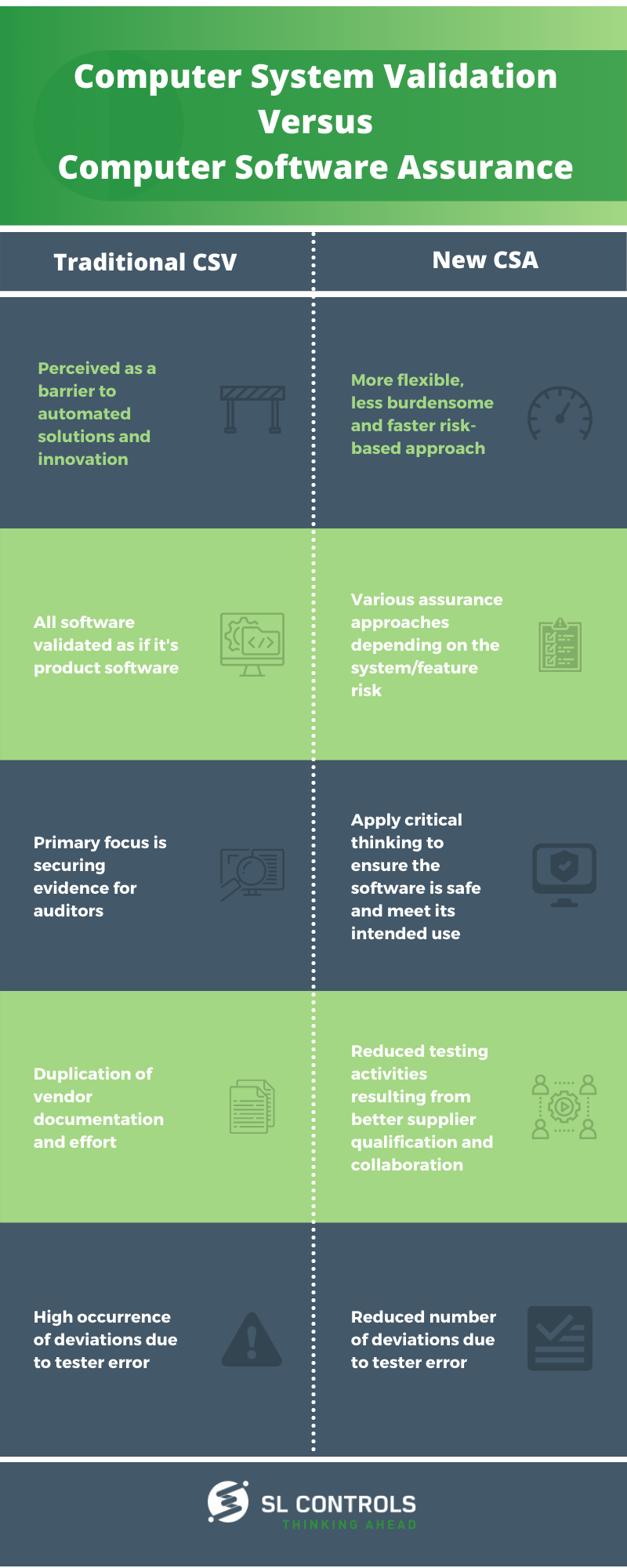
Infographic Computer System Validation Vs Computer Software Assurance Sl Controls

Computer Software Assurance Csa The New Csv

What S The Scoop On Fda S Upcoming Csa Computer Software Assurance Guidance Valimation
Infographic Computer System Validation Vs Computer Software Assurance Sl Controls
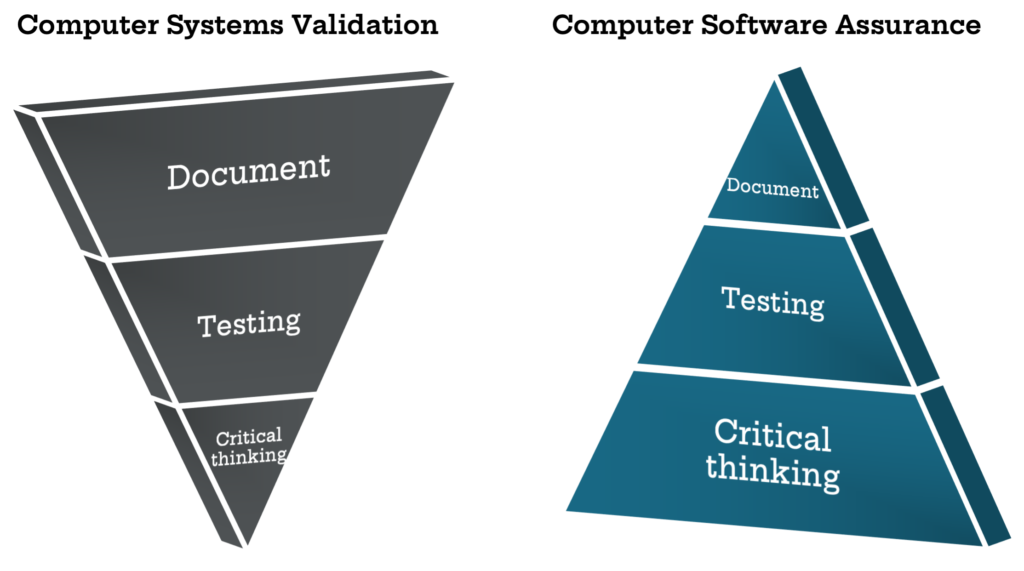
Computer Software Assurance Csa The Fda S New Approach To Csv Kalleid
Fda Computer System Software Validation What You Ve Known For 20 Years Is Changing

Critical Manufacturing Csa Vs Csv Fda S New Guidance For Software Assurance
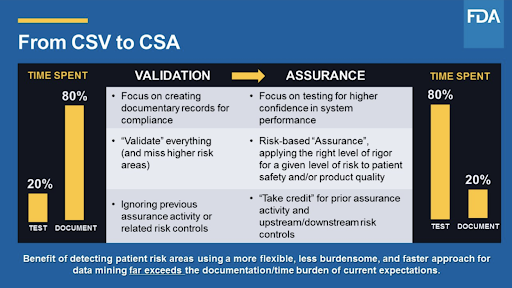
Fda Move From Software Validation To Computer Software Assurance Csa
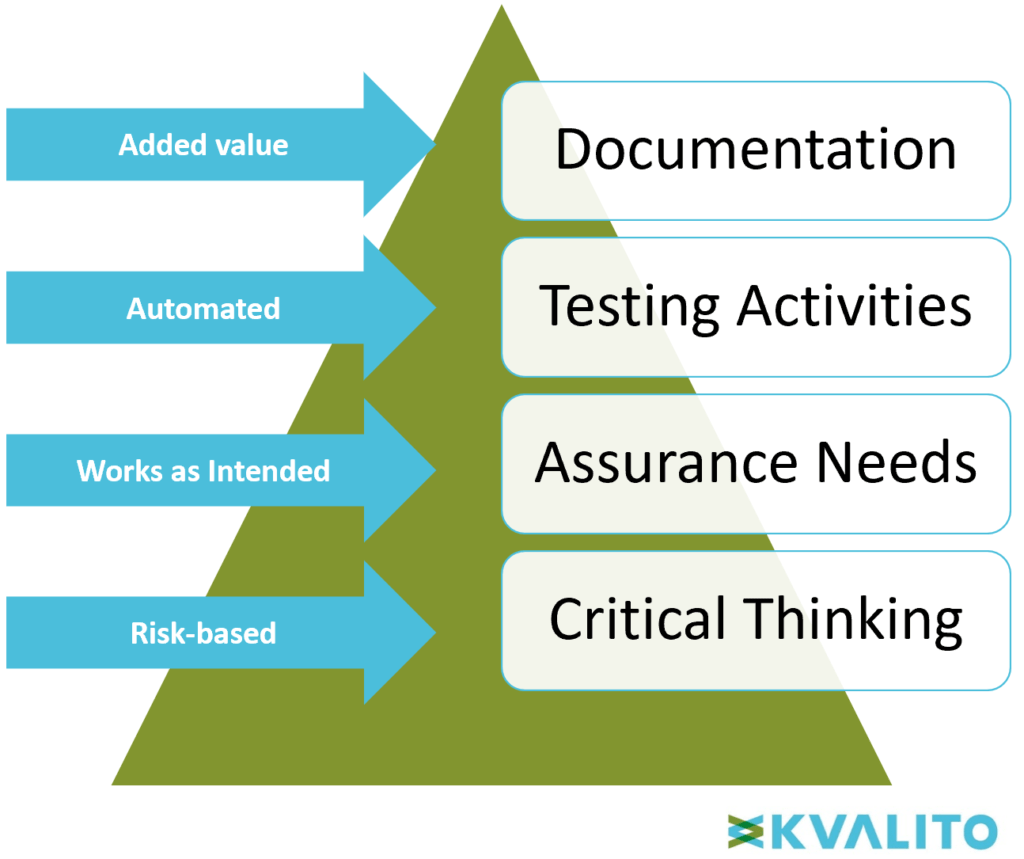
Risk Based Computerized System Validation Csv And Computer Software Assurance Csa Old Wine In A New Bottle Kvalito
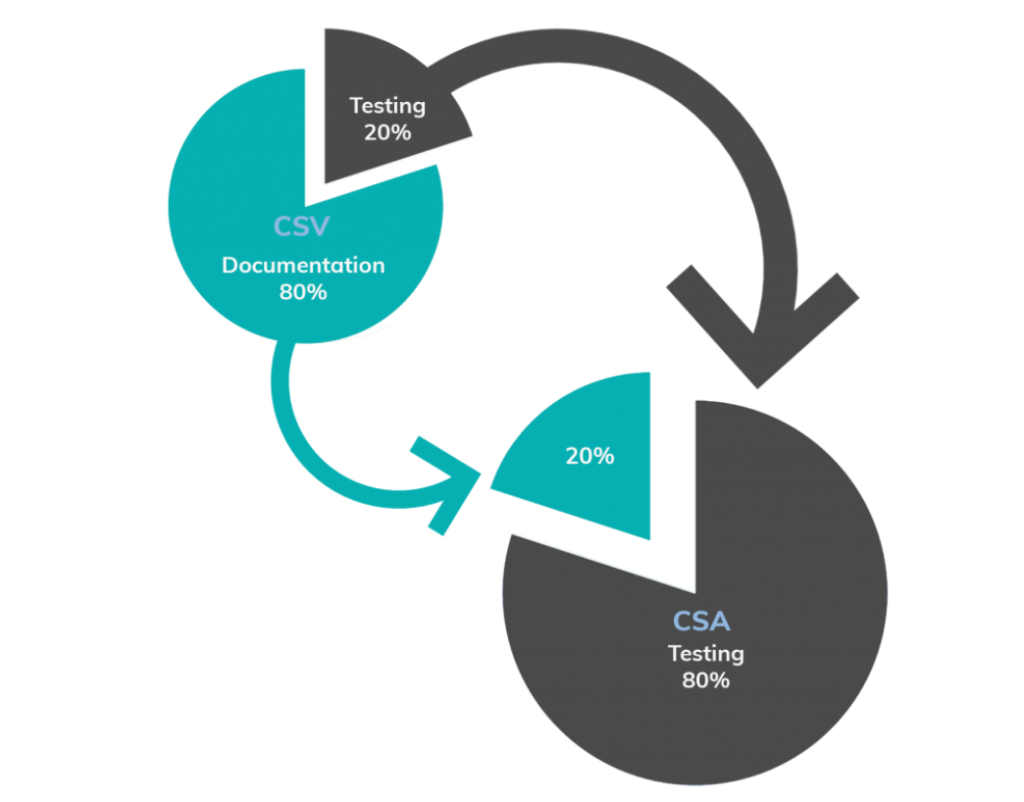
Critical Manufacturing Csa Vs Csv Fda S New Guidance For Software Assurance
![]()
Csa Computer Software Assurance A Move From Traditional Csv Amplelogic
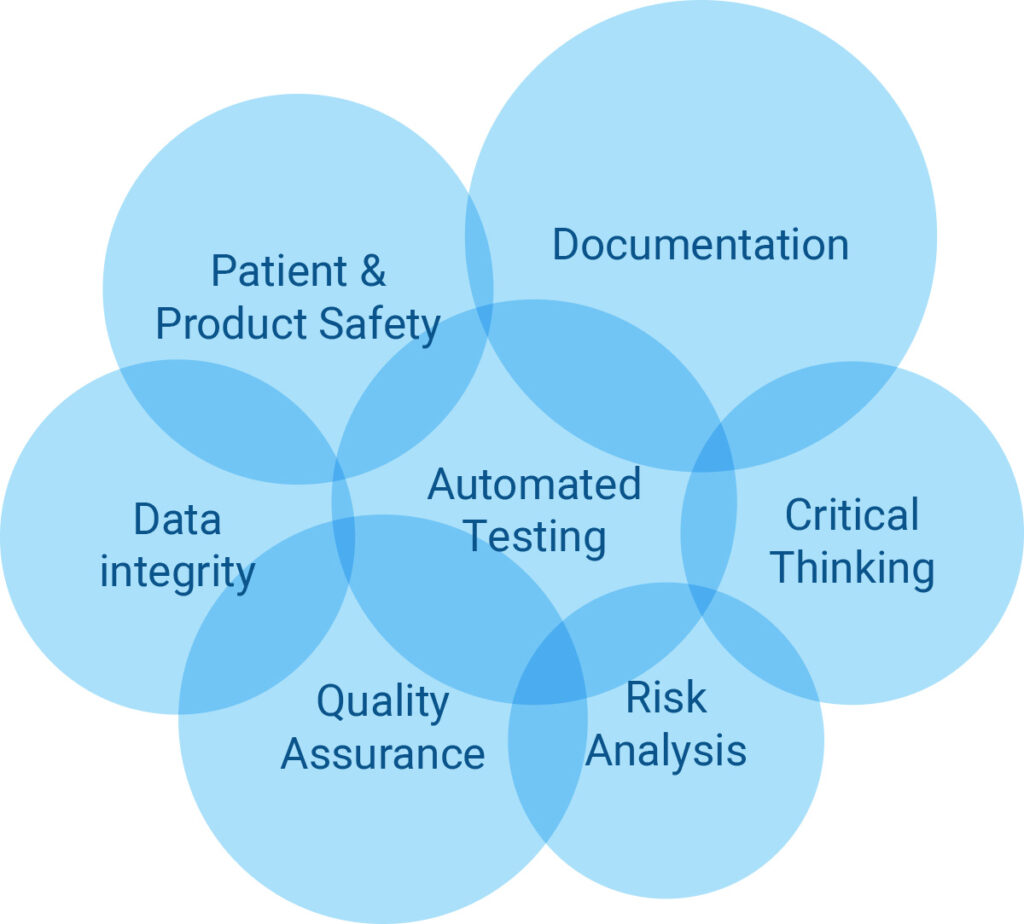
Critical Manufacturing Csa Vs Csv Fda S New Guidance For Software Assurance

Computer Systems Is Assurance The New Validation Quality By Design
Understanding Fda S Csa Guidance In The Context Of Current Regulations And Gamp American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology

Csa Computer Software Assurance A Move From Traditional Csv Amplelogic

Computer Software Assurance For Manufacturing Operations And Quality System Software Youtube
Posting Komentar untuk "Fda Computer System Assurance"