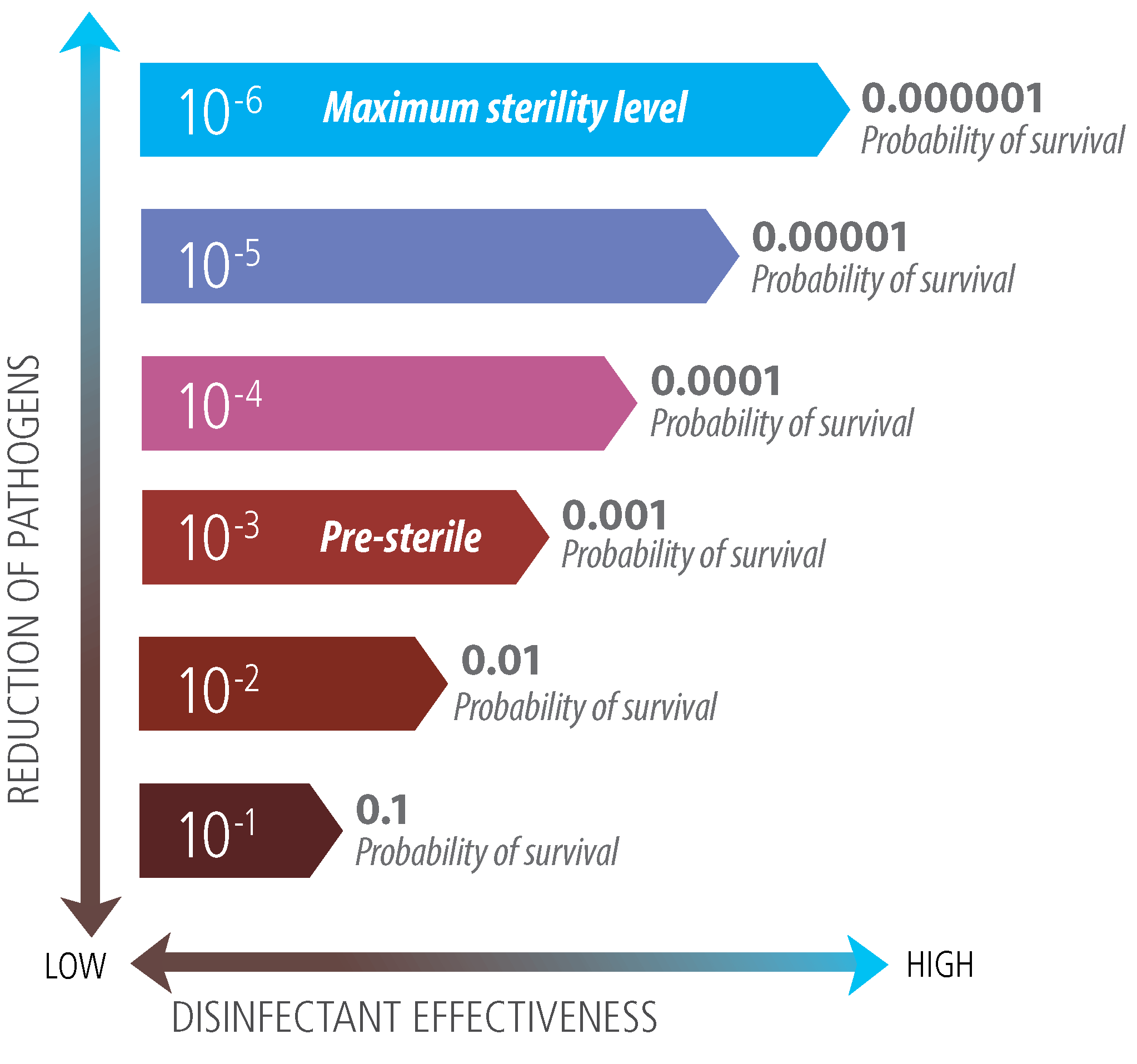
Sterility Assurance Level 10 6
Determination of sterility is often based on a sterility assurance level SAL. Higher than the maximum bioburden level expected on the product to be sterilized.

Simultaneous Achievement Of Sterility Assurance Level Sal Of 10 6 And Material And Functional Compatibility In Gas Plasma Sterilization Running Title Simultaneous Sal And Compatibility Semantic Scholar
While the probability can never be reduced to zero 100 assurance level and still have product for use it can be and is expected to be reduced to very low numbers.
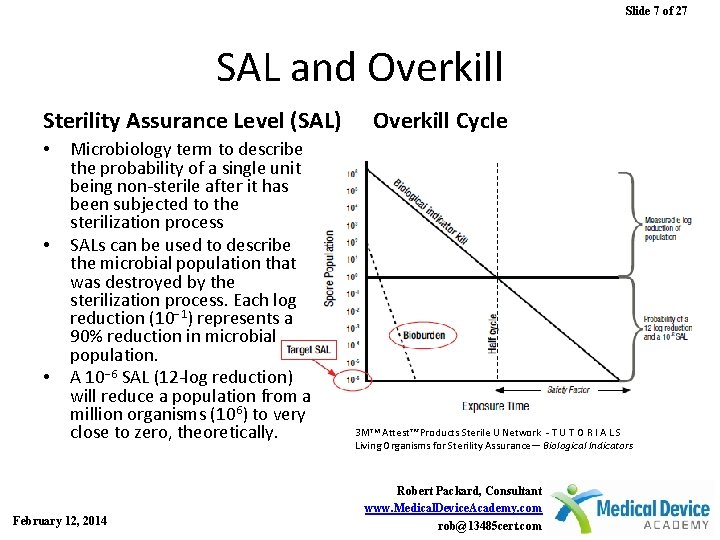
Sterility assurance level 10 6. Sterility means the absence of all viable microorganisms including viruses. The FDA requires devices labeled as sterile to have a SAL of 10 6 unless it is only intended for intact skin contact. Sterility achieved by filtration is not based on the same principles.
EN 556-1 specifies that a sterility assurance level SAL of 106 or less eg. For all sterility tests the test materials shall be supplied sealed in the original packaging meant for market distribution. Another Sterility assurance level may be applicable if the device offers superior benefits or there is no alternative product available in the market.
In which case a SAL of 10 3 is recommended. In such cases a sterility assurance level of greater than 10 6 eg. For example a 10-6 SAL is lower than a 10-3SAL and therefore provides a greater assurance of sterility.
In cases where bioburden level is elevated 1000 colony forming units or cfu per unit as may occur with very large single-use systems higher doses may be required to achieve sterility. For a healthcare product to be considered sterile the traditional options that have been accessible to a manufacturer are either terminal sterilization to a maximal sterility assurance level SAL of 10 6 or aseptic processing. Tim Sandle in Sterility Sterilisation and Sterility Assurance for Pharmaceuticals 2013.
At present a sterility assurance level SAL of 10 6 is generally accepted for pharmacopoeial sterilization procedures ie a probability of not more than one viable microorganism in an amount of one million sterilised items of the final product. At present a sterility assurance level SAL of 10-6 is generally accepted for pharmacopoeial sterilization procedures ie a probability of not more than one viable microorganism in an. 10 5 is considered through the risk assessment undertaken by.
While terminal sterilization is conducted on product after it is sealed in its sterile barrier packaging system aseptic processing involves the handling of. Devices purporting to be sterile attain a 106 microbial survivor probability ie. For aseptic processes a SAL is not applicable as accidental 56 contamination caused by inadequate technique cannot be reliably eliminated by monitor ing control or.
Based upon the bioburden of the product the defined sterilization process parameters will consistently be effective in obtaining a predetermined Sterility Assurance Level SAL. ISOTS 199302017 provides guidance on identifying the aspects to be considered as part of a risk-based approach to selecting a sterility assurance level SAL for terminally sterilized single-use health care product that is unable to withstand processing to achieve maximally a SAL of 106. SAL 10-6 D121 value x 14 SLR X minutes process exposure.
Any sterile medical device that may be sold in Europe must. Our sterility assurance level SAL is 10-6 and guarantees the less than 1 million possibility of a positive viable organism in our sterilized products. FDA regulation for a valid process and the products can be labeled sterile.
The sterilization process meets the US. A sterilizing membrane is composed of a multitude of pores that retain contaminants present in the. This is more accurate if F0 is used instead of simply minutes of exposure.
By extrapolating the reduction rates following extreme artificial. Generally 25 kGy can achieve sterility with a sterility assurance level SAL of 10 6. Generally in sterilization it is required to achieve a SAL sterility assurance level of 10 6 and an additional 6 log reduction.
54 provides a sterility assurance level SAL that is possible to calculate validate and control and thus 55 incorporates a safety margin. To accommodate for the variety in bioburden levels of Palls Allegro systems Verification Dose maximum VDmax sterilization validation approaches are used. Sterility means the absence of all viable microorganisms including viruses.
107 has to be achieved in order to designate a terminally sterilized medical device as sterile. Minimum Sterility Assurance Level. SAL or the Sterility Assurance Level is important in any production operation where there is a threat of contamination that could affect the product in the case of pharmaceutical manufacturing health of a.
An SAL of 10-6 is frequently used for the terminal sterilization. An SAL of 10-6 is frequently used for the terminal sterilization of. It means all 10 6 one million organisms present in the material are killed effectively and assured by extra 6 log reduction.
What is the difference between 10-5 and 10-6. Bioburden to the desired level and the impact of the sterilization process on the materials being. While the probability can never be reduced to zero 100 assurance level and still have product for use it can be and is expected to be reduced to very low numbers.
A 10-6 SAL is lower than a 10-3 SAL and therefore provides a greater assurance of sterility. Pyrogenicity Claim if applicable. Most medical devices are sterilized to achieve a SAL of 10-6 which is the probability of one in a million items being nonsterile.
Sterility Assurance James Agalloco Agalloco Associates Member USP Microbiology Sterility Assurance. Sterility assurance level SAL of 10-6 for devices labeled as sterile 10-3 for devices that only contact intact skin. A sterility assurance level SAL is defined as the probability of an item being nonsterile after it has been exposed to a validated sterilization process.
The minimum irradiation dose to obtain a sterility assurance level of 10-6 the prerequisite for a sterile claim depends on the average bioburden on the product to be sterilized. EN 556-1 includes an explanatory note that specifies that permission for acceptance of a sterility assurance level of greater than 106. The first part of this book described methods of terminal sterilisation where a product can be sterilised in its final container and different parametric attributes can be considered to assess the sterility assurance level and thus the probability of non-sterility can be assessed.

Simultaneous Achievement Of Sterility Assurance Level Sal Of 10 6 And Material And Functional Compatibility In Gas Plasma Sterilization Running Title Simultaneous Sal And Compatibility Semantic Scholar

Sterility Assurance Level Fda I3cglobal

Sterility Assurance Level An Overview Sciencedirect Topics

Medical Device Sterilization Pacific Bio Labs Inc 510

Sterility Assurance Level An Overview Sciencedirect Topics

D 10 Values Of Bacterial Isolates And Rsds Required For 10 6 Sterility Download Scientific Diagram
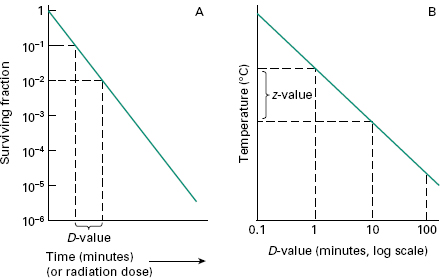
Sterilization Procedures And Sterility Assurance Basicmedical Key

Simultaneous Achievement Of Sterility Assurance Level Sal Of 10 6 And Material And Functional Compatibility In Gas Plasma Sterilization Running Title Simultaneous Sal And Compatibility Semantic Scholar

Sterilization And Validation Richard Marchand Md Medical Microbiologist And Infectious Diseases Assistant Professor University Of Montreal One Hospital Ppt Download

Simultaneous Achievement Of Sterility Assurance Level Sal Of 10 6 And Material And Functional Compatibility In Gas Plasma Sterilization Running Title Simultaneous Sal And Compatibility Semantic Scholar

Sterility Assurance Level An Overview Sciencedirect Topics

Pdf Validation Of Sterility Assurance Level Up To 10 6 Log Reductions
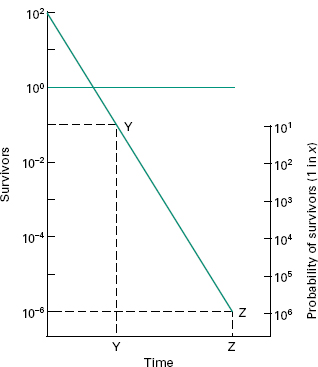
Sterilization Procedures And Sterility Assurance Basicmedical Key
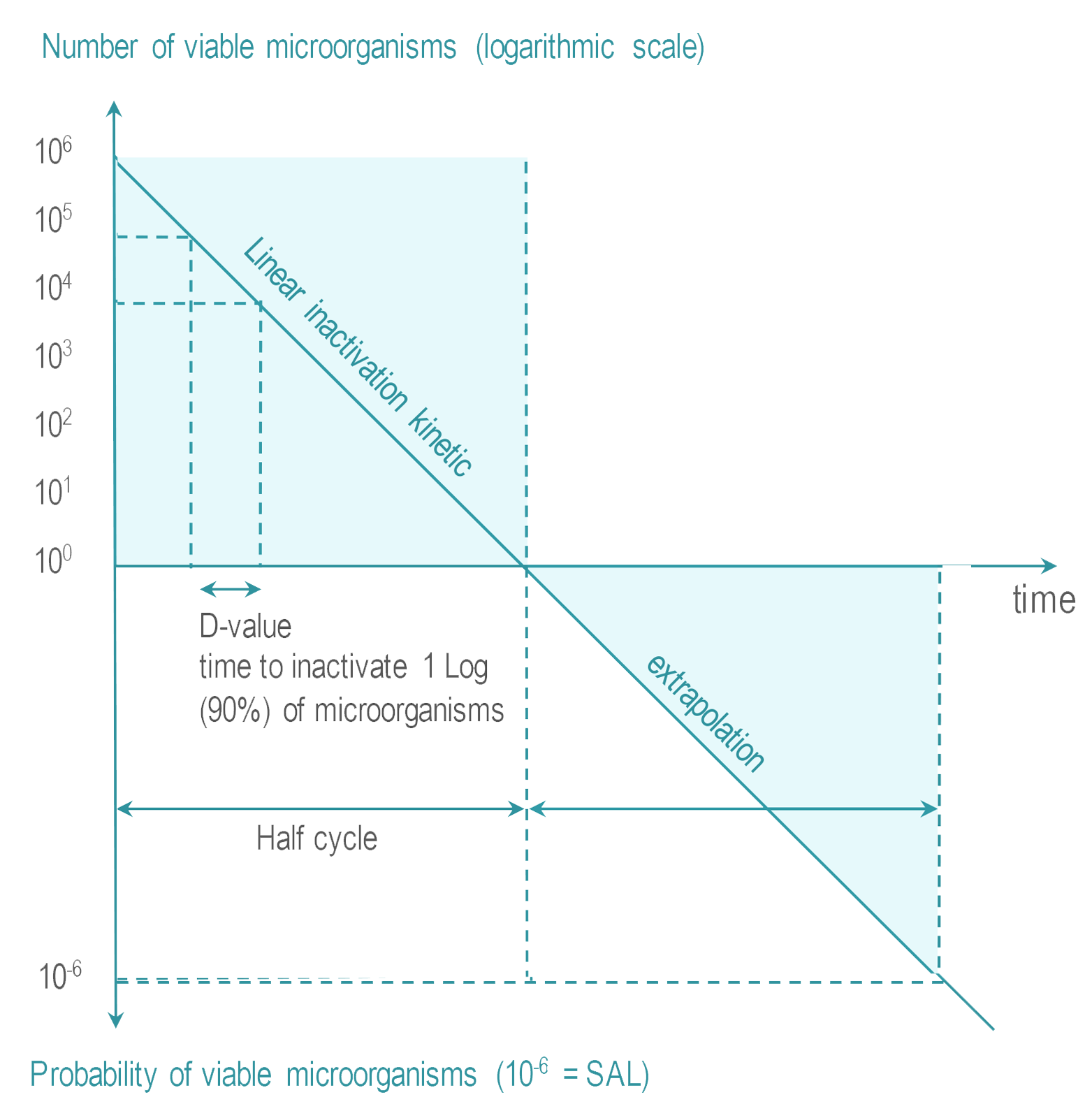
Sterilization Wfhss Guidelines

Slide 1 Of 27 Why Lowtemperature A Frozen
Posting Komentar untuk "Sterility Assurance Level 10 6"